XFOR: PDUFA Date of April 30, 2024 for Mavorixafor for WHIM Syndrome…
NASDAQ:XFOR
READ THE FULL XFOR RESEARCH REPORT
Business Update
PDUFA Date of April 30, 2024 for Mavorixafor for the treatment of WHIM Syndrome
In the fourth quarter of 2023, X4 Pharmaceuticals, Inc. (NASDAQ:XFOR) filed a New Drug Application (NDA) for mavorixafor for the treatment of patients 12 and older with WHIM (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) syndrome, a rare, primary immunodeficiency. The FDA granted Priority Review to the NDA and assigned a PDUFA action date of April 30, 2024. The company has indicated that Due to mavorixafor’s Rare Pediatric Disease designation for WHIM syndrome, X4 is eligible for a Priority Review Voucher (PRV) if mavorixafor is approved. PRV’s are fully transferable and a number of them have sold in the past few years for approximately $100 million each.
The company is currently focused on preparing for the potential approval of mavorixafor, including having discussion with payers and multiple meetings with the FDA to finalize the supply and distribution agreements in the event that the drug receives approval. X4’s commercial and medical affairs teams have grown along with brand marketing and the company’s sales infrastructure as it has increased its presence at medical meetings and engagements with hematologists and immunologists that are likely to have WHIM patients under their care. The company also reported that there has been strong engagement with the “What If It’s WHIM?” disease awareness campaign, with management stating there have been numerous reports of physicians thanking members of the X4 team for drawing their attention to WHIM syndrome, many of whom hadn’t heard of the disease and some thinking that perhaps they might have a WHIM patient under their care. Interactions like these may indicate that the approximately 1,000 WHIM patients estimated to be in the U.S. may be on the low end.
Update on Chronic CN Program
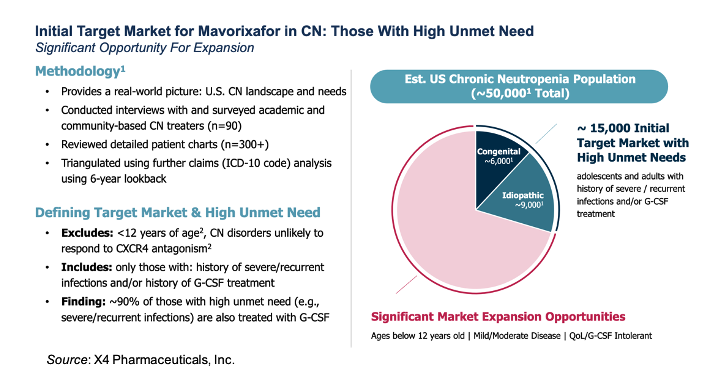
Chronic neutropenia (CN) is a disease that is defined as having severe, chronic (> 3 months) low levels of circulating neutrophils. Its etiology can be idiopathic (unknown origin), cyclic (neutrophil levels rise and fall in a cycle), or congenital (genetic cause). The following slide shows the results of a market survey conducted by X4 to better define the target market for mavorixafor in CN. It is estimated there are a total of approximately 50,000 CN patient’s in the U.S., with an initial target market of approximately 15,000 individuals with high unmet needs (adolescents and adults with severe/recurrent infections and/or G-CSF treatment).
X4 initially evaluated mavorixafor in a single-dose Phase 1b study in patients with CN. The rationale for testing mavorixafor in CN is due to neutrophil maturation, which occurs in the bone marrow, and mobilization out of the bone marrow being controlled by the CXCL12/CXCR4 signaling axis. The bone marrow contains approximately 20 times more neutrophils than are seen in circulation, and approximately 100 billion mature neutrophils are mobilized from the bone marrow each day (Furze et al., 2008). Maturation and mobilization of neutrophils results from downregulation of CXCR4, which decreases CXCR4 signaling, and antagonism of CXCR4 can also inhibit this signaling pathway (Mosi et al., 2012).
The Phase 1b CN trial was a success, as patients with all CN disorders (idiopathic, congenital, and cyclic) responded to mavorixafor treatment, with an increase in ANC of >2,000 cells/µL across all disorders. In severe neutropenia, all patients showed increased ANC to normal levels, which shows the potential for mavorixafor monotherapy. In addition, mavorixafor increased ANCs to normal levels in patients who were being treated with G-CSF, which supports further studies exploring the role of mavorixafor in replacing G-CSF therapy in these patients. Lastly, mavorixafor was well tolerated, all treatment-related adverse events were deemed to be low grade and consistent with what was seen in previous trials, and there were no treatment-related serious adverse events.
X4 is currently conducting a Phase 2 trial of mavorixafor in CN and previously announced preliminary data from the first three patients that completed the six-month trial in December 2023. The results showed that mavorixafor continues to be well tolerated when used in combination with G-CSF. The company achieved its target of at least 15 patients enrolled into the Phase 2 trial in early November 2023, and we anticipate additional results for the 15-plus trial participants in the coming months. That data is likely to include results from patients who received mavorixafor alone without G-CSF treatment.
The company completed the study design for a pivotal Phase 3 trial in CN, which we anticipate initiating in the first half of 2024. The following figure gives an overview of the study design, which will include approximately 150 subjects, a 12-month treatment period, and a two-component primary endpoint: annualized infection rate and ANC response.

Financial Update
On March 21, 20234, X4 announced financial results for the financial year ending December 31, 2023. As expected, the company did not report any revenues in 2023. R&D expenses for the year ending December 31, 2023 were $72.0 million, compared to $61.1 million for the year ending December 31, 2022. The increase in expenses was primarily due to a $5.0 million development milestone payment under the Genzyme agreement along with higher regulatory and consulting costs. SG&A expenses for the year ending December 31, 2023 were $35.5 million compared to $27.0 million for the year ending December 31, 2022. The increase was primarily due to increased compensation expense and higher stock-based compensation costs.
As of December 31, 2023, X4 had cash, cash equivalents, marketable securities, and restricted cash of approximately $115.2 million. We estimate that the company has sufficient capital to fund operations into 2025. This projection does not include any potential drawdowns from the company’s debt facility with Hercules Capital or the potential monetization of the PRV.
As of March 18, 2024, X4 had approximately 167.9 million shares outstanding and, when factoring in stock options and warrants, a fully diluted share count of 291.7 million.
Conclusion
We are eagerly anticipating the PDUFA date of April 30, 2024 for mavorixafor for the treatment of WHIM syndrome. If the drug is approved, we fully anticipate a PRV being issued along with it and would expect the company to monetize the PRV in a short amount of time. We look forward to additional data from the company’s Phase 2 trial of mavorixafor in CN along with the initiation of the Phase 3 CN trial. We have advanced our DCF model ahead by one year which has resulted in an increase to our valuation to $5.00 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
